Publications
Publications
* indicates co-first authors; ^ indicates co-corresponding authors
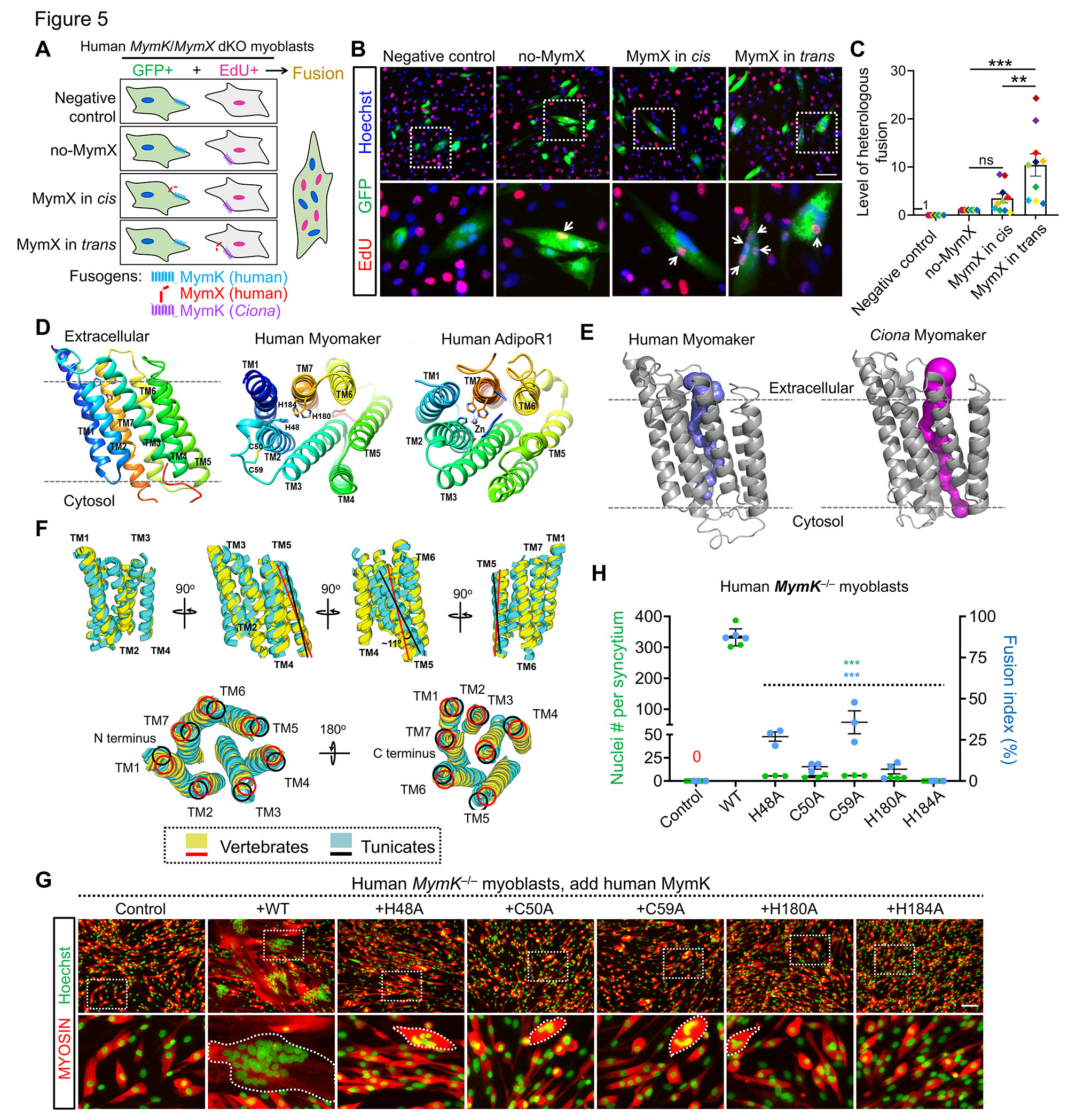
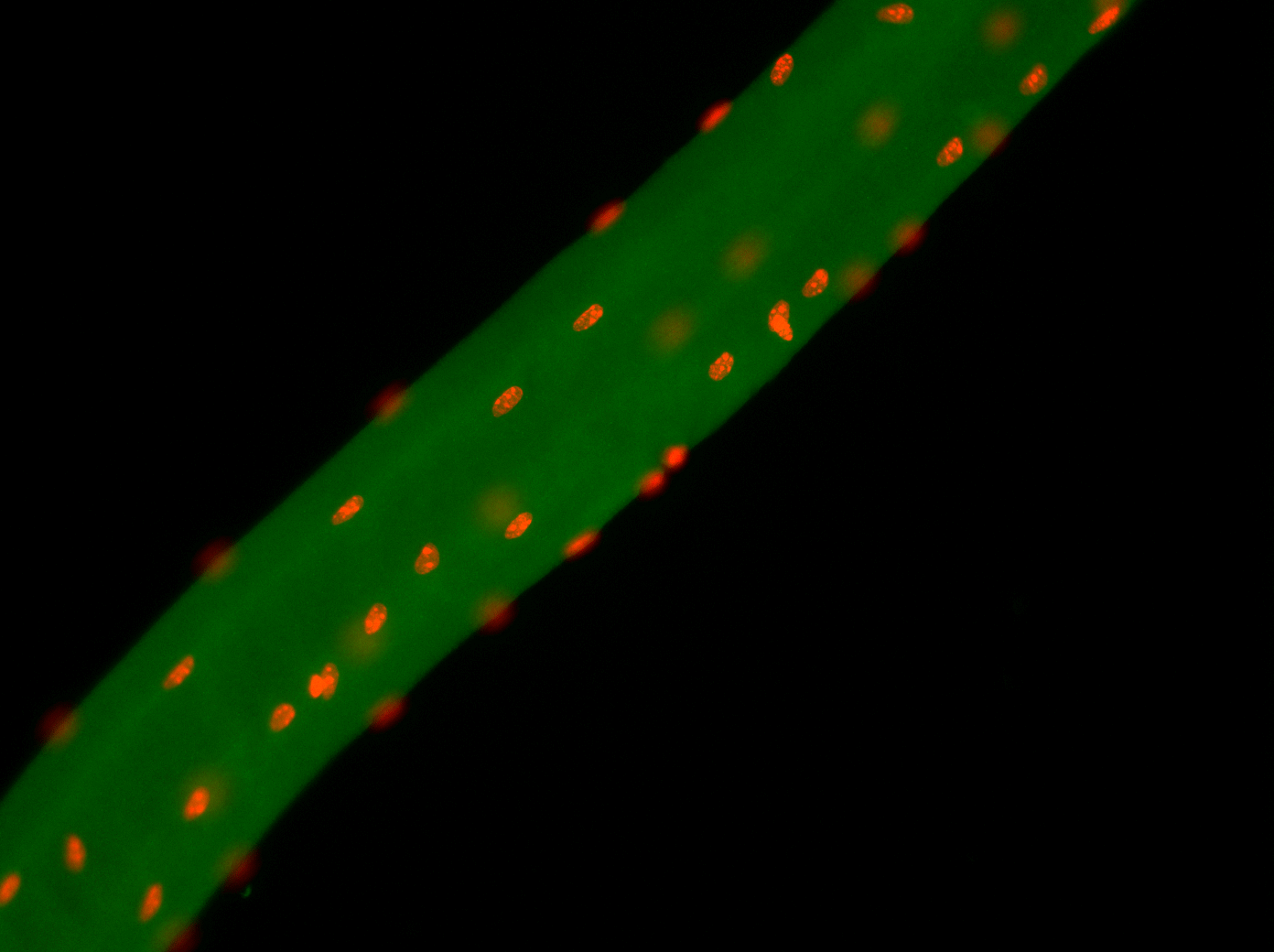
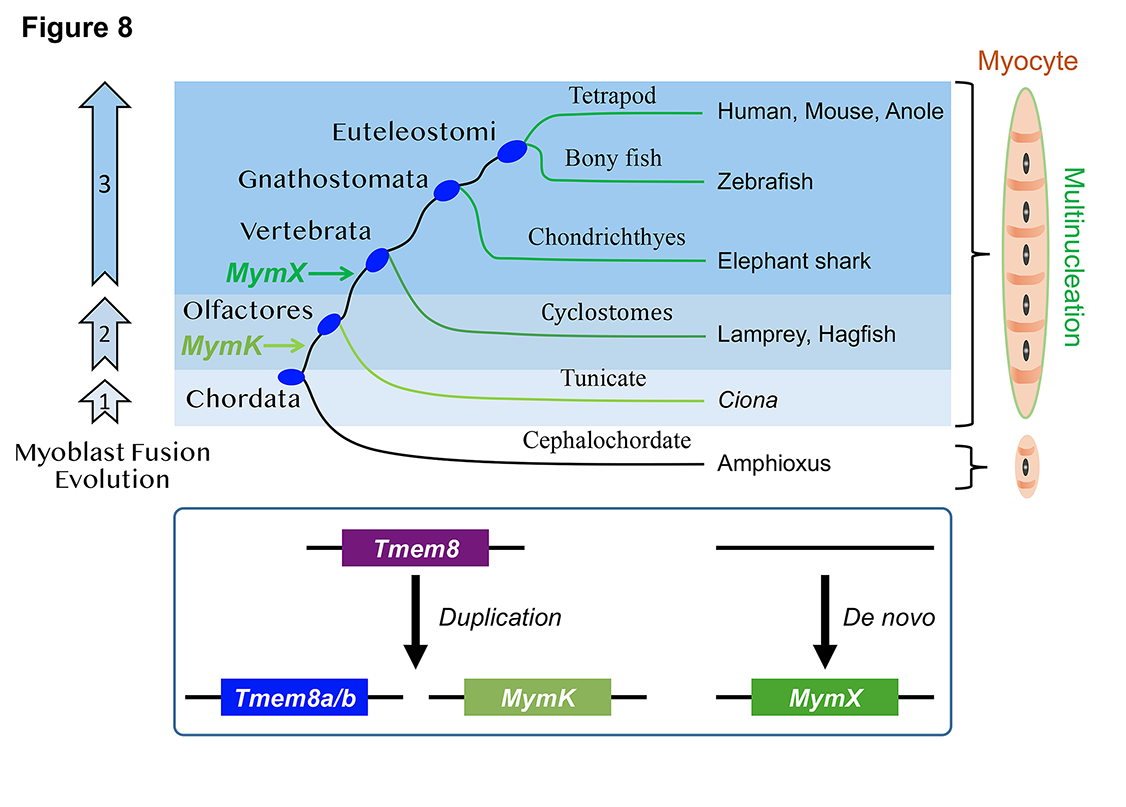
25. Zhang H, Shang R, Kim K, Zheng W, Johnson CJ, Sun L, Niu X, Liu L, Zhou J, Liu L, Zhang Z, Uyeno TA, Pei J, Fissette SD, Green SA, Samudra SP, Wen J, Zhang J, Eggenschwiler J, Menke D, Bronner ME, Grishin NV, Li W, Ye K, Zhang Y, Stolfi A^, Bi P^. Evolution of a chordate-specific mechanism for myoblast fusion. Science Advances. 2022 Sep 2;8(35):eadd2696.
24. Zhang H, Shang R, Kim K, Zheng W, Johnson CJ, Sun L, Niu X, Liu L, Uyeno TA, Zhou J, Liu L, Pei J, Fissette SD, Green SA, Samudra SP, Wen J, Zhang J, Eggenschwiler J, Menke D, Bronner ME, Grishin NV, Li W, Ye K, Zhang Y, Stolfi A^, Bi P^. Evolution of a chordate-specific mechanism for myoblast fusion. bioRxiv. 24 July 2021. doi: https://doi.org/10.1101/2021.07.24.453587
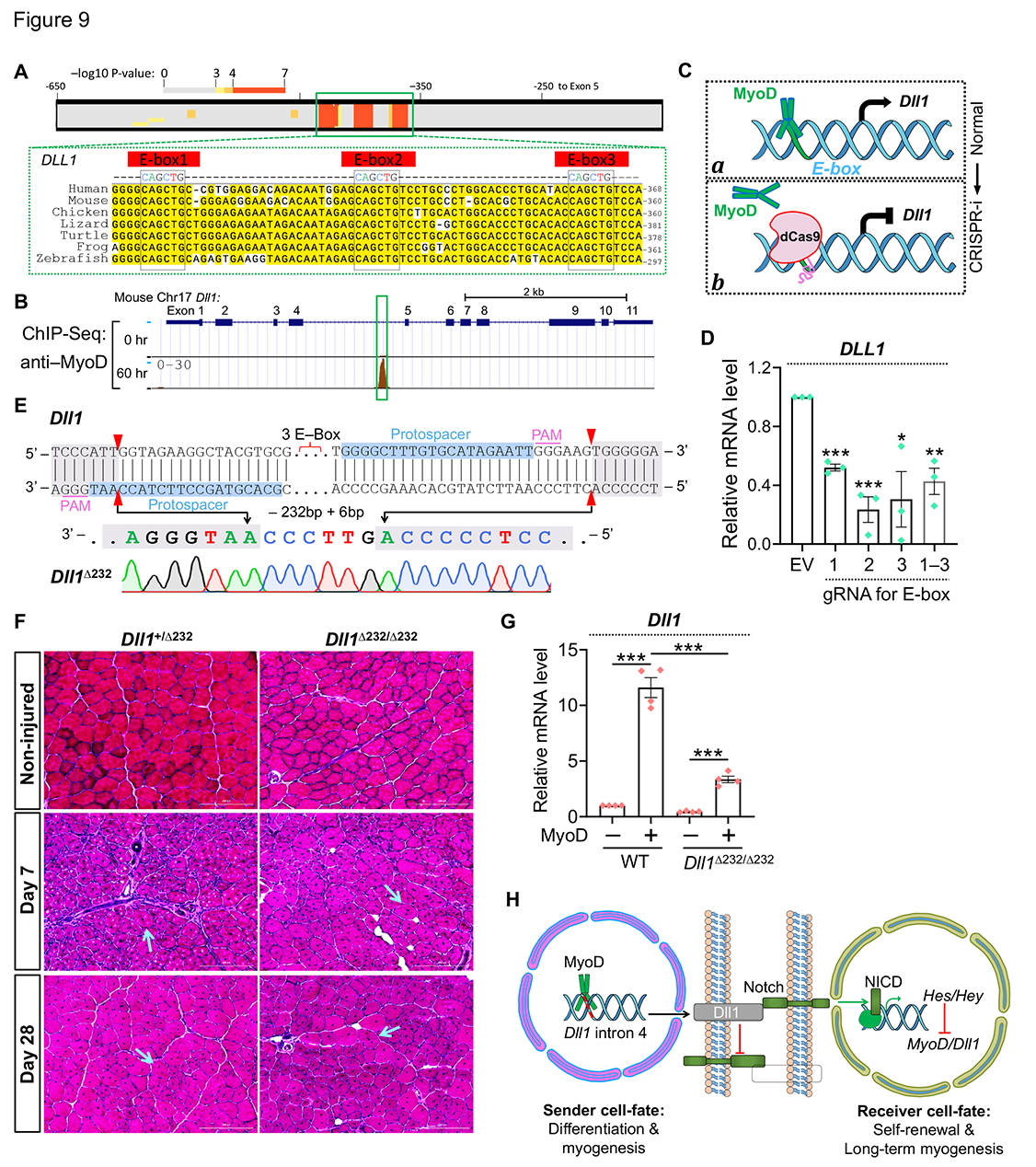
23. Zhang H, Shang R, Bi P^. Feedback regulation of Notch signaling and myogenesis connected by MyoD–Dll1 axis. PLoS Genetics. 2021 Aug 9;17(8):e1009729.
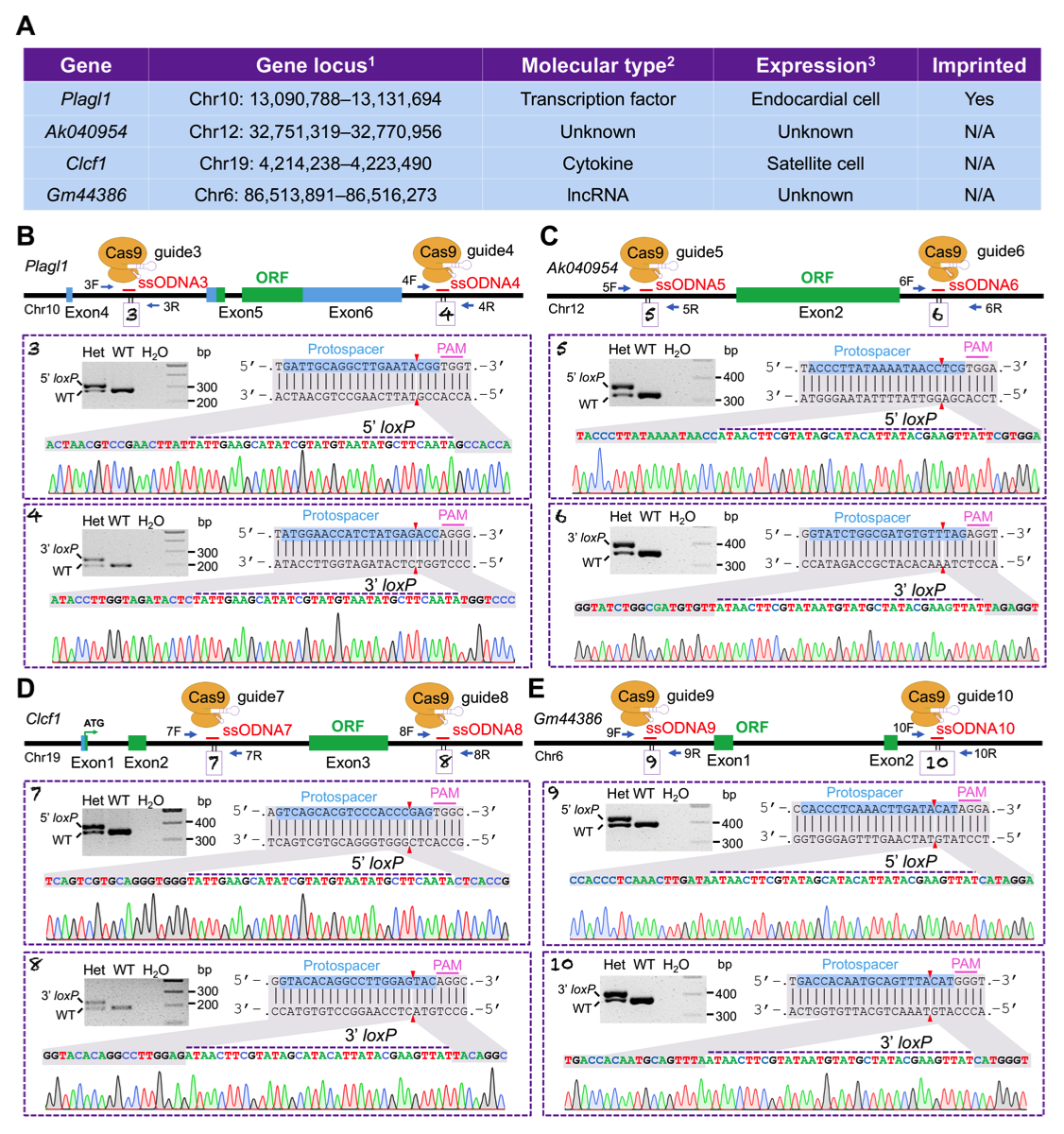
22. Shang R, Zhang H, Bi P^. Generation of mouse conditional knockout alleles in one step using the i-GONAD method. Genome Research. 17 December 2020
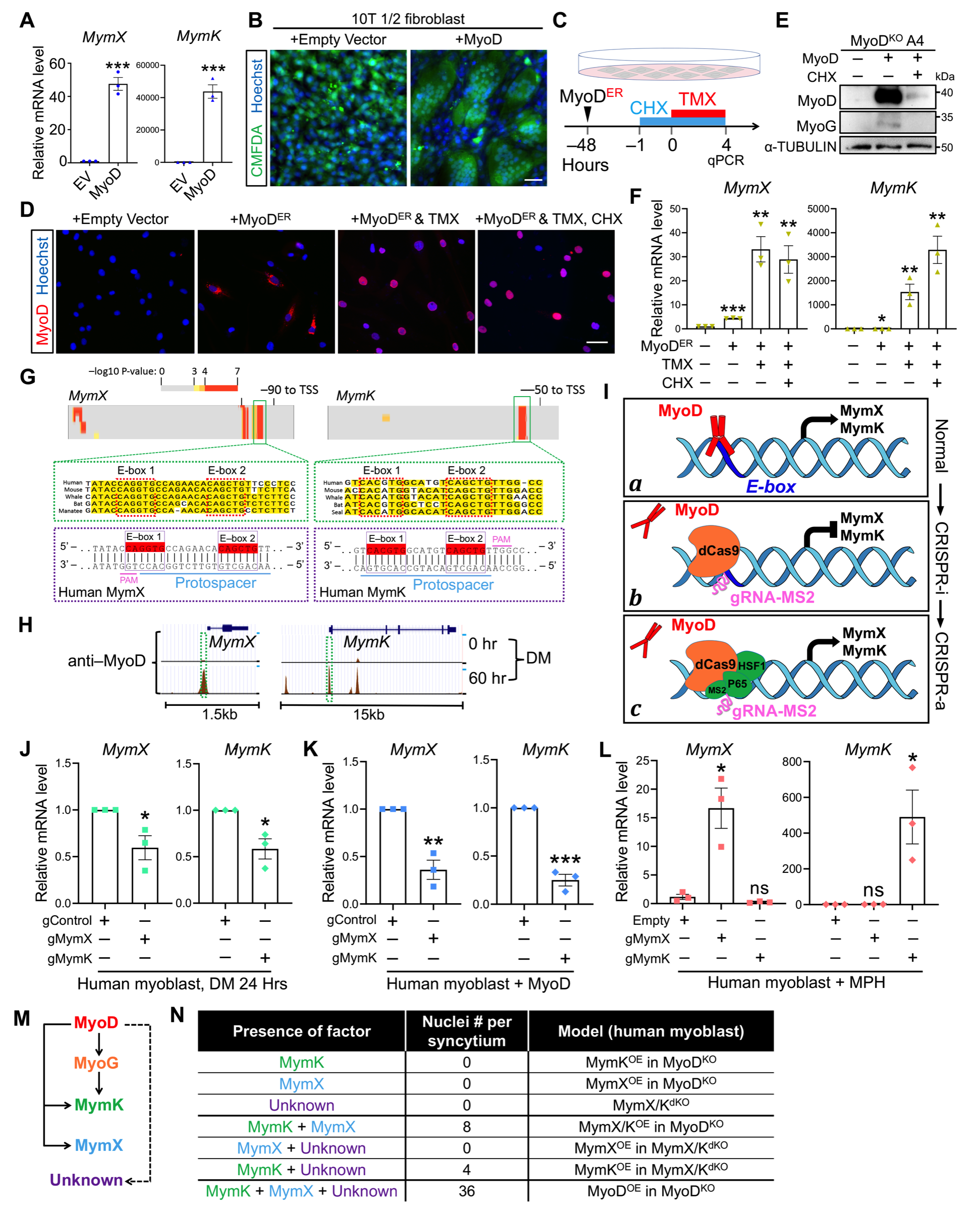
21. Zhang H, Wen J, Bigot A, Chen J, Shang R, Mouly V, Bi P^. Human myotube formation is determined by MyoD–Myomixer/Myomaker axis. Science Advances. 2020; 6 : eabc4062 18 December 2020
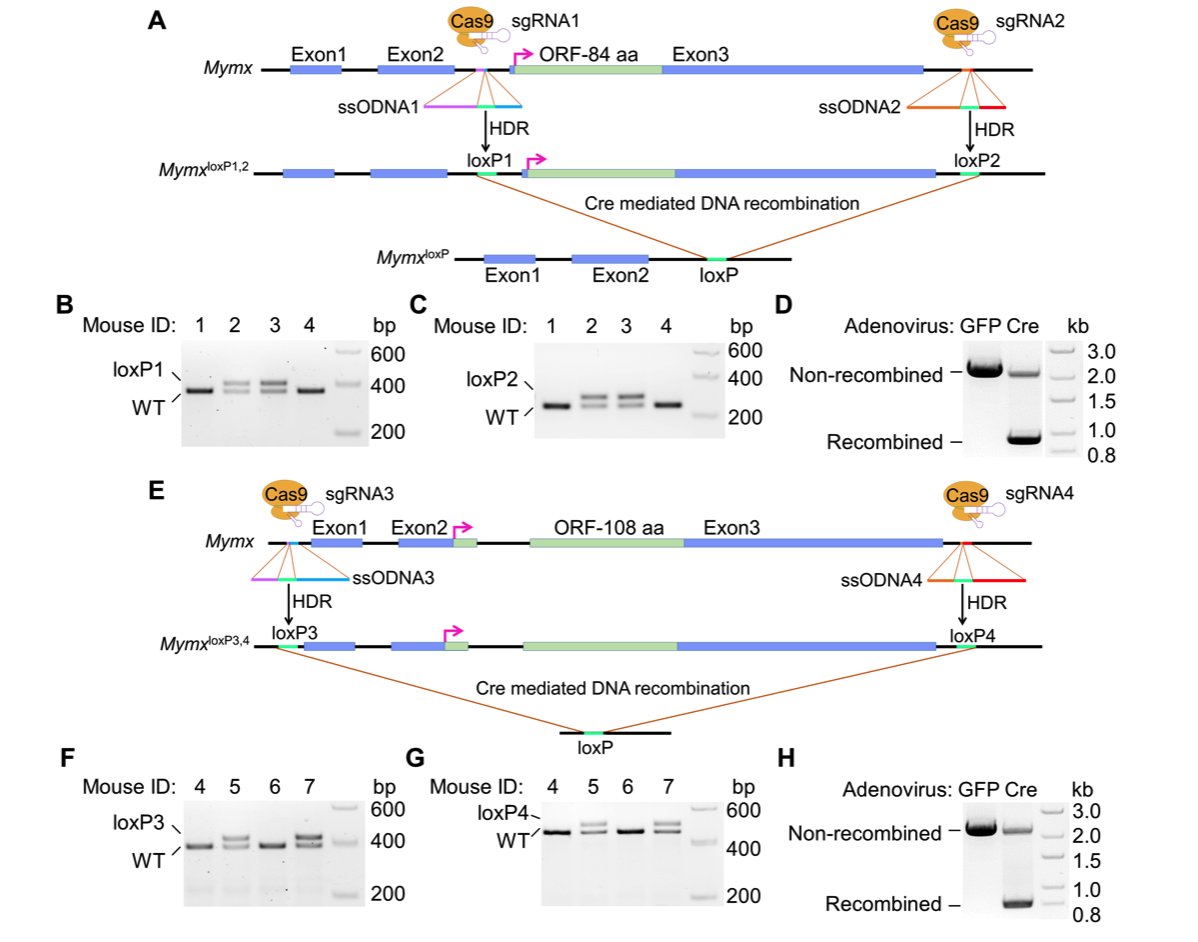
20. Bi P, McAnally JR, Shelton JM, Sánchez-Ortiz E, Bassel-Duby R and Olson EN. The fusogenic micropeptide Myomixer is essential for satellite cell fusion and muscle regeneration. PNAS. 2018; 115(15): 3864-3869.
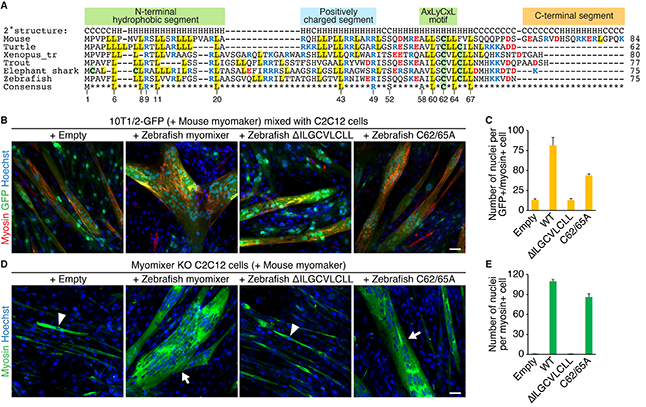
19. Shi J*, Bi P*, Pei J, Li H, Grishin NV, Bassel-Duby R, Chen EH, Olson EN. Requirement of the fusogenic micropeptide myomixer for muscle formation in zebrafish. PNAS. 2017; 114(45): 11950-11955.
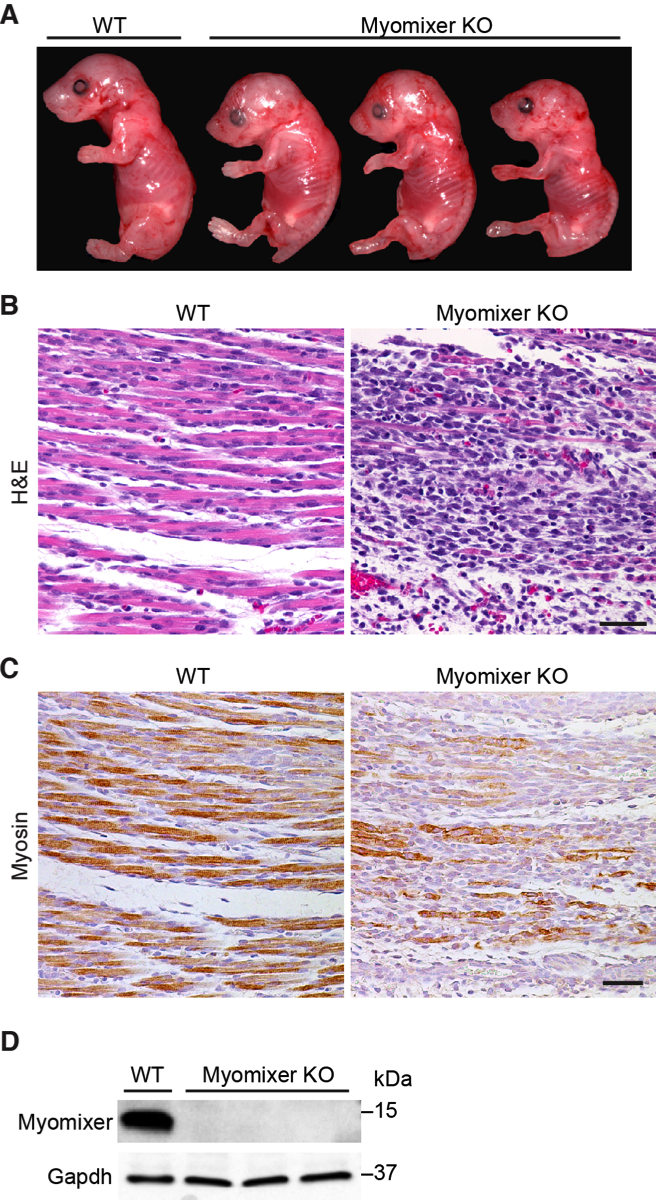
18. Bi P, Ramirez-Martinez A, Li H, Cannavino J, McAnally JR, Shelton JM, Sánchez-Ortiz E, Bassel-Duby R, Olson EN. Control of muscle formation by the fusogenic micropeptide myomixer. Science. 2017; 356(6335): 323-327.
Main Text Supplementary Materials
Press Coverage: Muscular Dystrophy News, ScienceDaily, MEMBS, F1000 Prime
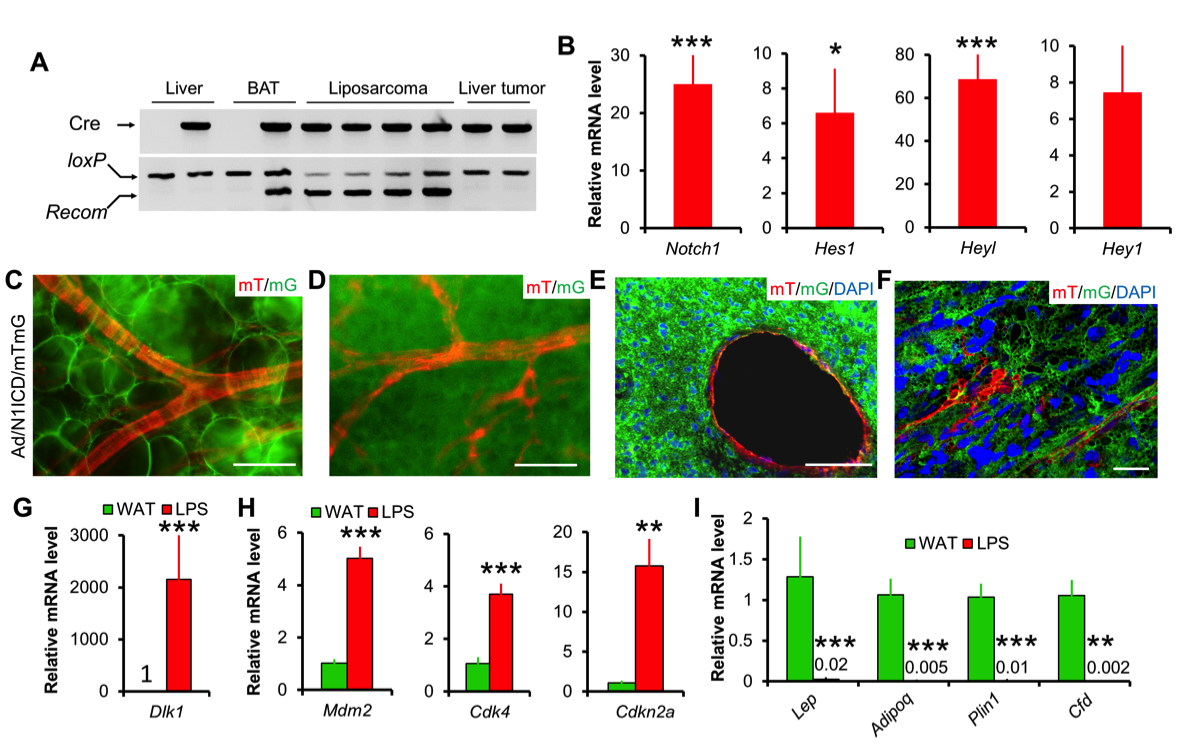
17. Bi P^, Yue F, Karki A, Castro B, Wirbisky SE, Wang C, Durkes A, Elzey BD, Andrisani OM, Bidwell CA, Freeman JL, Konieczny SF, Kuang S. Notch activation drives adipocyte dedifferentiation and tumorigenic transformation in mice. J Exp Med. 2016; 213(10):2019-37.
Press Coverage: ScienceDaily, Medical Xpress, EurekAlert.
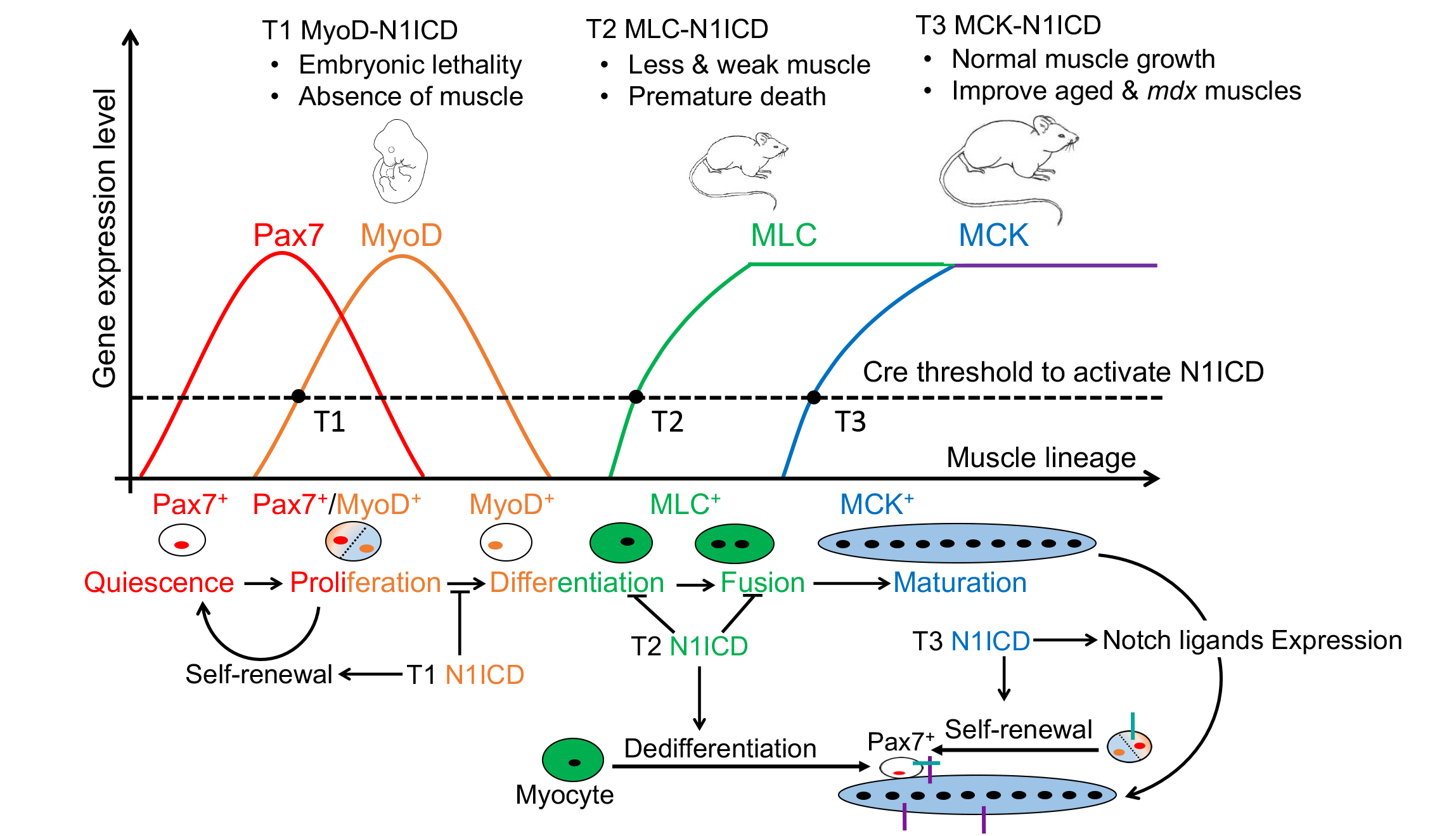
16. Bi P*, Yue F*, Sato Y, Wirbisky S, Liu W, Shan T, Wen Y, Zhou D, Freeman J, Kuang S. Stage-specific effects of Notch activation during skeletal myogenesis. eLife. 2016; e17355.
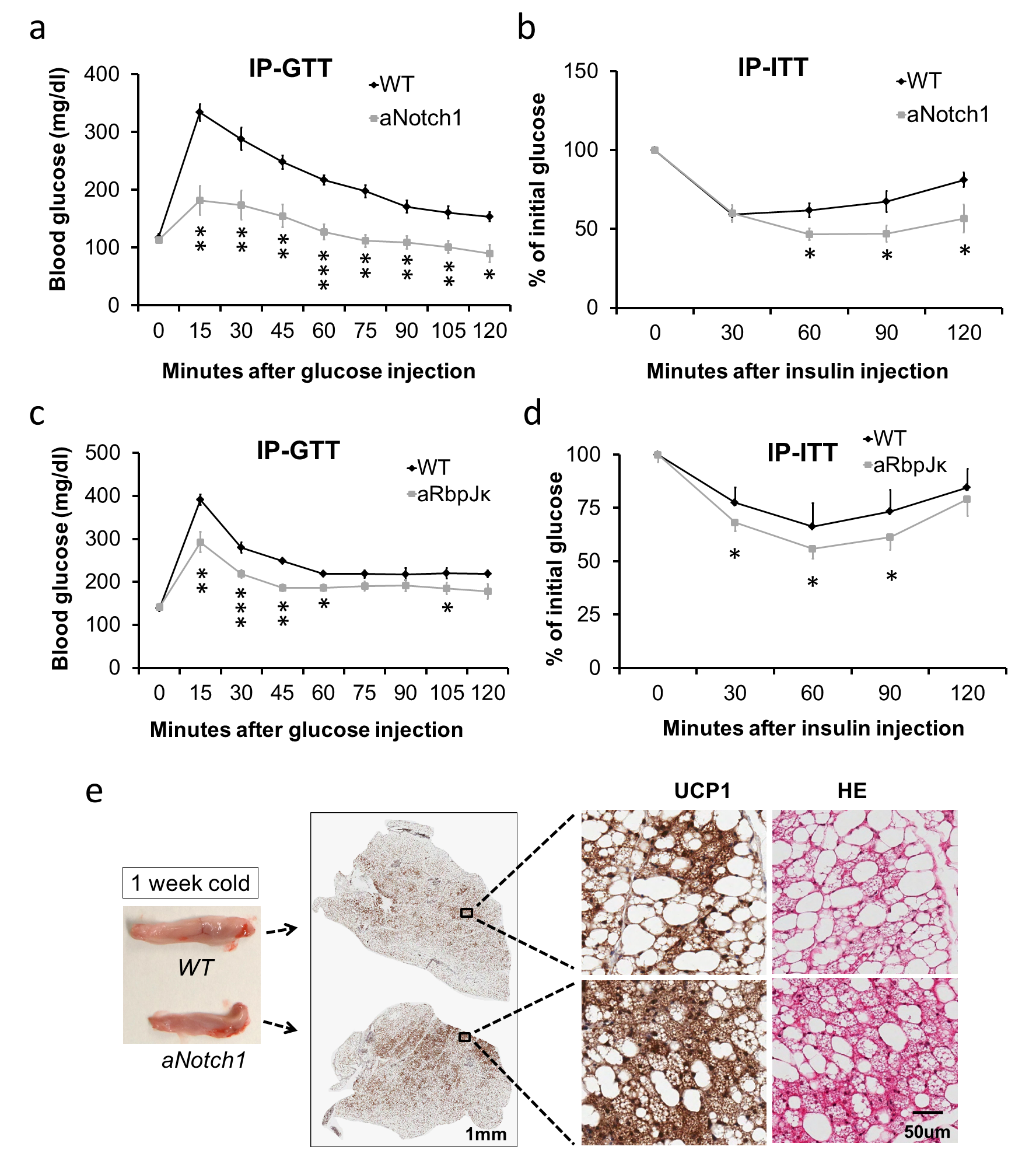
15. Bi P, Shan T, Liu W, Yue F, Yang X, Liang XR, Wang J, Li J, Carlesso N, Liu X, Kuang S. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med. 2014. 20(8):911-918.
Main Text Supplementary Materials
Highlighted in: Nature Medicine, Science Signaling.
Press Coverage: Innovations Report, ScienceDaily, HealthCanal, R&D.
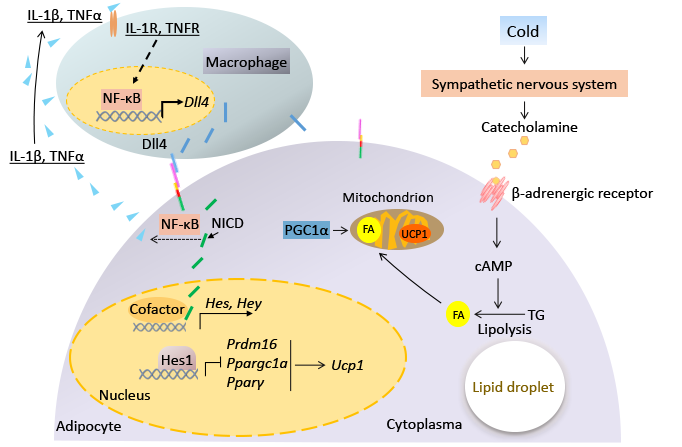
14. Bi P^, Kuang S. Notch signaling as a novel regulator of metabolism. Trends Endocrinol Metab. 2015; 26(5): 248-55.
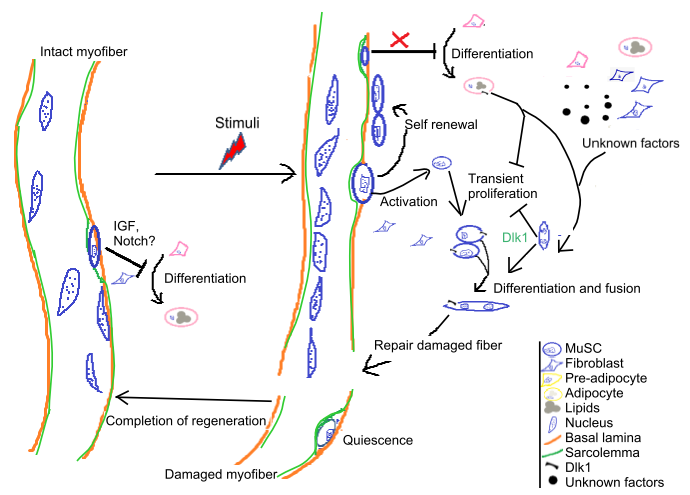
13. Bi P, Kuang S. Meat Science and Muscle Biology Symposium: stem cell niche and postnatal muscle growth. J Anim Sci. 2012; 90(3):924-35.
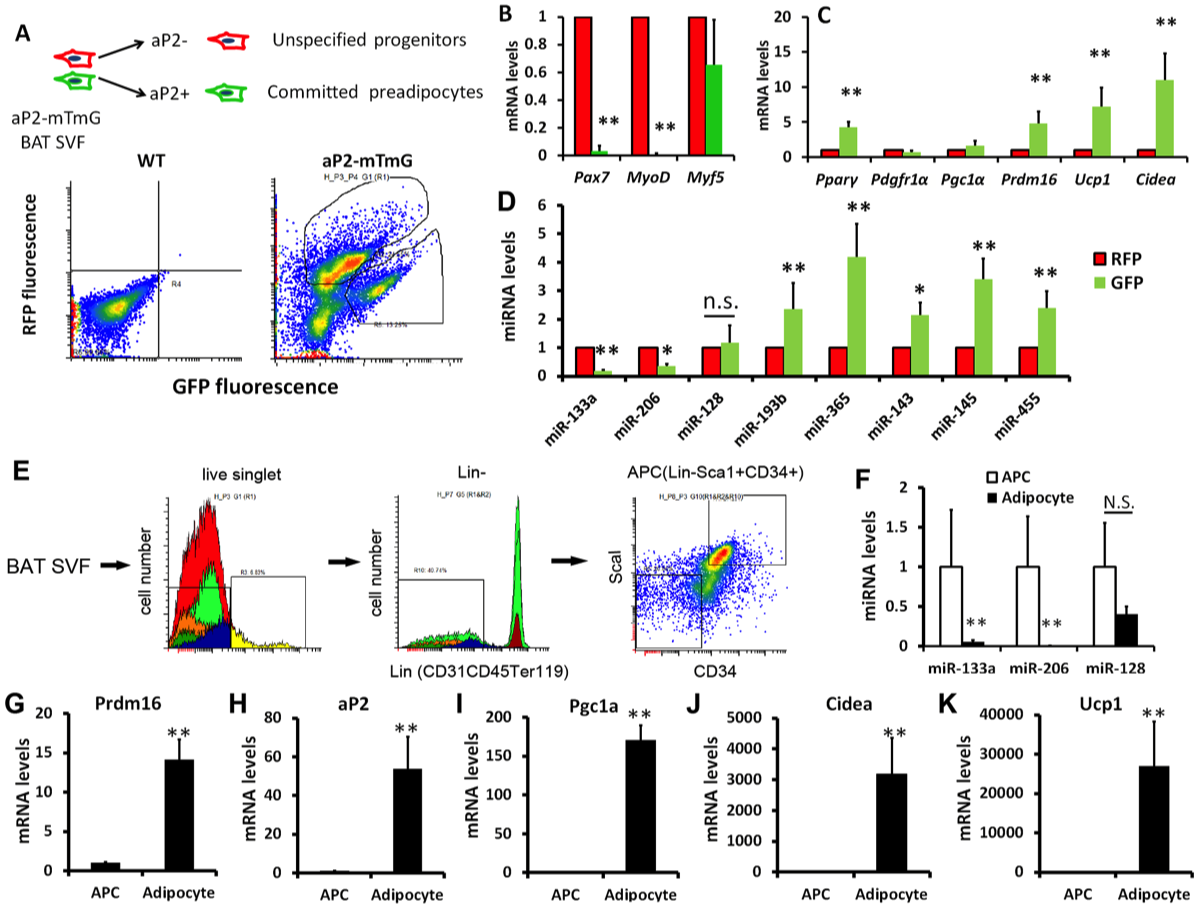
12. Liu W*, Bi P*, Shan T, Yang X, Yin H, Wang YX, Liu N, Rudnicki MA, Kuang S. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013; 9(7) :e1003626.

11. Yue F, Bi P, Wang C, Li J, Liu X, Kuang S. Conditional loss of Pten in myogenic progenitors leads to postnatal skeletal muscle hypertrophy but age-dependent exhaustion of satellite cells. Cell Rep. 2016; 17(9): 2340-2353.

10. Yue F, Bi P, Wang C, Shan T, Nie Y, Ratliff TL, Gavin TP, Kuang S. Pten is necessary for the quiescence and
maintenance of adult muscle stem cells. Nat Commun. 2017; 8:14328.

9. Wen Y, Bi P, Liu W, Asakura A, Keller C, Kuang S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol. 2012; 32(12):2300-11.

8. Li Z, Li J, Bi P, Lu Y, Burcham G, Elzey BD, Ratliff T, Konieczny SF, Ahmad N, Kuang S, Liu X. Plk1 phosphorylation of PTEN causes a tumor-promoting metabolic state. Mol Cell Biol. 2014; 34(19):3642-61.

7. Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, Kuang S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development. 2012; (16):2857-65.

6. Yang X, Bi P, Kuang S. Fighting obesity: When muscle meets fat. Adipocyte. 2014; 3(4):280-9.

5. Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK- PGC1α-Fndc5 pathway in muscle. FASEB J. 2013; 27(5): 1981-9.

4. Shan T, Liang X, Bi P, Zhang P, Liu W, Kuang S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res. 2013; 54(8): 2214-24.

3. Shan T, Zhang P, Bi P, Kuang S. Lkb1 deletion promotes ectopic lipid accumulation in muscle progenitor cells and mature muscles. J Cell Physiol. 2015; 230(5):1033-41.

2. Shan T, Zhang P, Liang X, Bi P, Yue F, Kuang S. Lkb1 is indispensable for skeletal muscle development, regeneration, and satellite cell homeostasis. Stem Cells. 2014; 32(11): 2893-907.

1. Shan T, Xiong Y, Zhang P, Li Z, Jiang Q, Bi P, Yue F, Yang G, Wang Y, Liu X, Kuang S. Lkb1 controls brown adipose tissue growth and thermogenesis by regulating the intracellular localization of CRTC3. Nat Commun. 2016; 7:12205.